Source: Genprex, Inc. 1/4/2022
Pipeline Expansion Enables Company to Target Entire Lung Cancer Market with REQORSA™
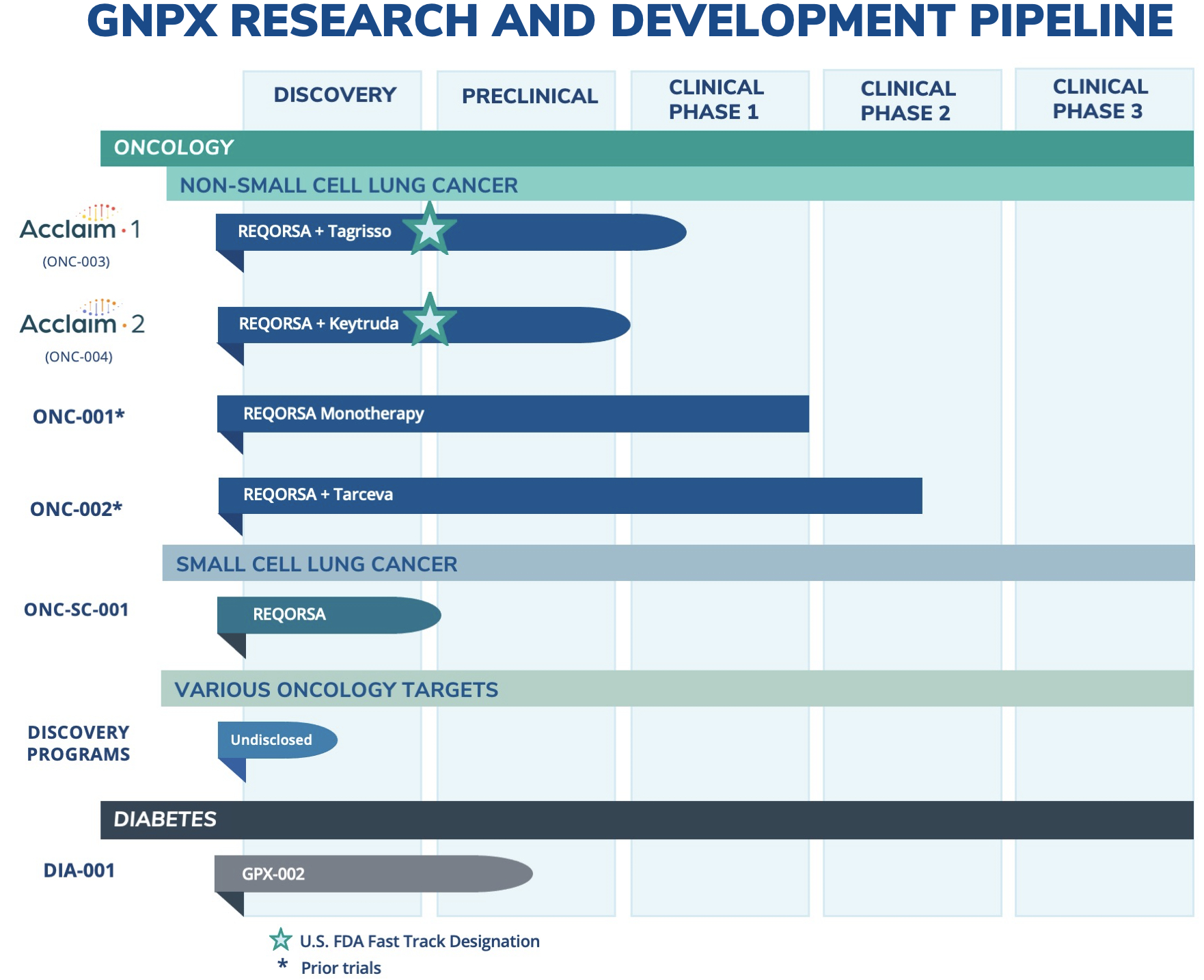
Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that it has expanded its oncology research and development pipeline to include small cell lung cancer (SCLC) as an additional disease indication for its lead drug candidate, REQORSA™ Immunogene Therapy. SCLC represents approximately 10-15 percent of the lung cancer market, while REQORSA’s initial target indication of non-small cell lung cancer (NSCLC) represents approximately 84 percent of the lung cancer market.
“Data from human studies indicate that REQORSA could be beneficial in targeting small cell lung cancer,” said Mark S. Berger, MD, Chief Medical Officer at Genprex. “Like non-small cell lung cancer, small cell lung cancer consistently has low TUSC2 protein levels and is documented to often have deletion of one TUSC2 gene allele. Extensive stage small cell lung cancer has a very poor prognosis, with a median progression free survival of 5.2 months. Expanding the therapeutic indications targeted by REQORSA to include small cell lung cancer may provide us with another important clinical opportunity to combine REQORSA with small cell lung cancer therapies, including checkpoint inhibitors.”
REQORSA consists of a TUSC2 gene expressing plasmid encapsulated in non-viral lipid nanoparticles. Genprex’s oncology program utilizes its unique, proprietary, non-viral ONCOPREX® Nanoparticle Delivery System, which the Company believes is the first systemic gene therapy delivery platform used for cancer in humans. The ONCOPREX Nanoparticle Delivery System could allow for the delivery of a number of cancer-fighting genes, alone or in combination with other cancer therapies to potentially address unmet medical need in cancer, thereby potentially improving patient outcomes through the advancement of multiple therapeutic approaches for large patient populations.
“REQORSA may have the potential to treat small cell lung cancer, in addition to non-small cell lung cancer, thus making REQORSA a possible drug candidate for the entire lung cancer market,” said Rodney Varner, Genprex’s Chief Executive Officer. “Lung cancer continues to be the leading cause of cancer deaths worldwide, causing more deaths than colorectal, breast, liver or stomach cancers. We hope that one day REQORSA will provide improved outcomes for virtually all lung cancer patients.”
About Genprex, Inc.
Genprex, Inc. is a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes. Genprex’s technologies are designed to administer disease-fighting genes to provide new therapies for large patient populations with cancer and diabetes who currently have limited treatment options. Genprex works with world-class institutions and collaborators to develop drug candidates to further its pipeline of gene therapies in order to provide novel treatment approaches. Genprex’s oncology program utilizes its unique, proprietary, non-viral ONCOPREX® Nanoparticle Delivery System, which the Company believes is the first systemic gene therapy delivery platform used for cancer in humans. ONCOPREX encapsulates the gene-expressing plasmids using lipid nanoparticles. The resultant product is then administered intravenously, where it is then taken up by tumor cells that express proteins that are deficient. The Company’s lead product candidate, REQORSA™ (quaratusugene ozeplasmid), is being evaluated as a treatment for non-small cell lung cancer (NSCLC). REQORSA has a multimodal mechanism of action that has been shown to interrupt cell signaling pathways that cause replication and proliferation of cancer cells; re-establish pathways for apoptosis, or programmed cell death, in cancer cells; and modulate the immune response against cancer cells. REQORSA has also been shown to block mechanisms that create drug resistance. In 2020, the U.S. Food and Drug Administration (FDA) granted Fast Track Designation for REQORSA for NSCLC in combination therapy with AstraZeneca’s Tagrisso® (osimertinib) for late-stage patients with EFGR mutations whose tumors progressed after treatment with Tagrisso. In 2021, the FDA granted Fast Track Designation for REQORSA for NSCLC in combination therapy with Merck & Co’s Keytruda® (pembrolizumab) for late-stage patients whose disease progressed after treatment with Keytruda.

No comments:
Post a Comment